Air and Atmosphere Selina Concise Class 7 Chemistry Chapter 7
*Bonus Question:- Do you know the difference between Air and Atmosphere?
Click here to know the answer!
Part B: Short/Long Answer Type Questions
Part A: OBJECTIVE TYPE QUESTIONS
1. Fill in the blanks : (Answers in bold)
(a) Argon is the most abundant inert gas present in air.
(b) Oxides of sulphur and nitrogen combine with rain water to form sulphuric acid and nitric acid which cause acid rain.
(c) Nitrogen dioxide and CO are the most common air pollutants.
(d) Joseph Priestly discovered the oxygen gas.
(e) Oxygen occupies about 21% of air by volume.
2. Match the following :
3. MULTIPLE CHOICE QUESTIONS (Answers in Bold)
1. A fuel when used releases least amount of pollutants in the air.
(a) sulphur dioxide
(b) chlorofluorocarbon
(c) smoke
(d) CNG
2. The natural way of adding oxygen to air which involves green plants is called
(a) photosynthesis
(b) respiration
(c) burning
(d) dissolution
3. Which one of the following is most likely to be corroded?
(a) a stainless steel cup-board
(b) a galvanised iron bucket
(c) an iron hammer
(d) a tin plated iron box
4. The process by which oxidation of food in our body takes place is
(a) photosynthesis
(b) respiration
(c) decomposition
(d) combustion
Part B: Short/Long Answer Type Questions
A: Air: A Mixture of Gases
Exercise I
Question 1.
Give one use for each of the following inert gases :
(a) argon
(b) helium
(c) neon
(d) radon
(e) krypton
(f) xenon
Answer:
(a) Argon— Argon is filled into electric bulbs to prevent the oxidation of their filaments.
(b) Helium— It is used in filling up weather observation balloons.
(c) Neon— Neon is used for making advertisement sign boards.
(d) Radon— It is used for treatment of Cancer.
(e) Krypton— It is used in photography.
(f) Xenon— It is also used in photography.
Question 2.
Answer the questions put against each of the following constituents of air :
(a) Nitrogen : Explain its significance for plants and animals.
(b) Oxygen : What is the percentage proportion of oxygen in air ? Why is oxygen called active air.
(c) Carbon dioxide : “Although carbon dioxide plays no role in respiration, all life would come to an end if there is no carbon dioxide in air.” Support this statement with relevant facts.
(d) Water vapours : Explain their role in modifying the earth’s climate.
Answer:
(a) Plants convert nitrogen into protein. It is an important constituent of proteins, which are necessary for the growth of animals, plants and human beings. Plants convert nitrogen into proteins.
(b) 20.9%, oxygen is called active air because it supports life on earth. It is essential for the process of combustion.
(c) Carbon dioxide is essential for photosynthesis by which green plants prepare their food. It minimises heat loss by radiation. Thus, it balances the temperature on earth.
(d) Water vapour determine the earth’s climate conditions. It causes rain. It controls the rate of evaporation from the bodies of plants and animals.
Question 3.
Define the following terms :
(a) pollutants
(b) acid rain
(c) Global warming
(d) smog
Answer:
(a) Pollutants : Air contains substances which are harmful to plants and animals. These harmful substances are called pollutants.
(b) Acid rain : When sulphur trioxide and nitrogen oxide present in the air mix with rainwater they form sulphuric acid and nitric acid respectively. Rainwater containing these acids is called acid rain.
(c) Global warming : An increase in the percentage of carbon dioxide, methane, nitrous oxide and chlorofluorocarbon traps the heat causing the temperature of the earth and its surroundings to rise. This is known as global warming.
(d) Smog : Oxides of nitrogen form a mixture of smoke and fog known as smog which affects our eyes too.
Question 4.
“Air is a mixture”. Support this statement citing at least three evidences.
Answer:
“Air is a mixture” The following are in evidences which prove that air is a mixture.
1. The composition of air varies from place to place and from time to time.
2. The components of air retain their individual properties.
3. Liquid air has no definite boiling point.
4. No energy exchange occurs when the components of air are mixed with each other.
Question 5.
What is air pollution ? What are the harmful effects of sulphur dioxide, nitrogen dioxide and hydrogen sulphide present in the air ?
Answer:
Air Pollution : Air is polluted by natural processes like volcanic eruption, crop pollination, etc. mostly it is polluted by human activities like burning of coal, wood, diesel oil, kerosene, petrol etc.
Fossil fuels contain sulphur and nitrogen as impurities.
When fuels bum these substances combine with air to produce gasses like sulphur dioxide, nitrogen oxide and hydrogen sulphide. They cause many serious respiratory problems. They can destroy the ozone layer, which protects us from the ultra violet radiations of the Sun. They also cause acid rain, which damages crops and buildings.
Question 6.
(a) What are the causes of air pollution ?
(b) Suggest five measures to prevent air pollution.
Answer:
(a) When fuels bum they produce sulphur dioxide, sulphur trioxide, nitrogen dioxide, hydrogen sulphide when these gases mix with rain water. They produce sulphuric and nitric acid. These acids mix with rain water to form acid rain.
(b) Five measures for the prevention of air pollution are:
1. By using smokeless sources of energy, like solar energy and electrical energy, in place of conventional fossil fuels.
2. By using filters for the. smoke coming out of the chimneys of factories and power plants.
3. By using internal combustion engines in vehicles for complete and efficient burning of fuel.
4. By locating industries away from residential areas.
5. By growing more trees.
Question7.
(a) What is nitrogen-fixation ?
(b) What are the two ways in which nitrogen fixation occurs?
(c) Explain the conversion of nitrogen into nitrates during lightning.
Answer:
(a) Nitrogen fixation : Nitrogen fixation is the process by which nitrogen present in the atmosphereis converted into nitrogen compounds useful for other biochemical processes such as absorption of plant.
(b) i) Biological Fixation
ii) Non-biological Fixation.
(c) During lightning, temperatures often reach as high as 3000°C. At such high temperatures, nitrogen and oxygen present in the air combine to form nitric oxide, which further react with oxygen to form nitrogen dioxide:
Nitrogen dioxide then reacts with the water vapour present in air to form nitrous and nitric acids.
Nitric acid, so formed, reaches the earth along with rain-water, and reacts with metal carbonates to form metal nitrates.
B. OXYGEN
EXERCISE — II
Question 1.
Name :
(a) The most abundant element in the earth’s crust.
Ans. Oxygen.
(b) A chemical called oxygenated water.
Ans.Hydrogen peroxide
(c) A metal highly resistant to rusting.
Ans. Tin.
(d) A mixture of oxygen and carbon dioxide used for artificial respiration.
Ans. Carbogen
(e) Two substances from which oxygen can be obtained at a large scale.
Ans. Air, water.
(f) An oxide and a carbonate containing oxygen.
Ans. Mercuric oxide and potassium chlorate.
(g) Two substances which undergo rapid oxidation.
Ans. Sodium, carbon.
Question 2.
(a) Taking hydrogen peroxide, how would you prepare oxygen in the laboratory ?
(b) What is the role of manganese dioxide in the preparation of oxygen ?
(c) Write the balanced chemical equation for the above chemical reaction.
(d) Why is hydrogen peroxide preferred in the preparation of oxygen gas ?
(e) Why is oxygen collected by downward displacement of water ?
(f) What happens when a glowing splinter is introduced in a jar containing oxygen ?
(g) What happens when oxygen gas is passed through alkaline pyrogallol solution ?
Answer:
(a) Take manganese dioxide in a round bottom flask and add hydrogen peroxide drop by drop to it, which acts a catalyst as shown in the figure. Collect oxygen by downward displacement of water.
(b) Manganese dioxides acts as a catalyst.
(d) Hydrogen peroxide is preferred for lab preparation of oxygen because of following reasons:-
i)No heating is required.
ii)The rate of evolution of oxygen is moderate and under control.
iii)It is a safe chemical.
(e) Since the water is displaced downward by the gas collecting in the jar, the process is called downward displacement of water. The reasons are :
i)Oxygen is only slightly soluble in water. Therefpre it can be collected over water without fear of excessive dilution.
ii)Oxygen is slightly heavier than air, so it cannot collected over air.
(f) The glowing splinter rekindles, but the gas does not catch fire.
(g) Alkaline pyrogallol solution turns brown when oxygen is passed through it.
Question 3.
(a) What happens when
1. mercuric oxide and
2. potassium nitrate are heated ?
(b) Why is potassium chlorate not used for laboratory preparation of oxygen ?
Answer:
(a)
1. When mercuric oxide is heated, it decomposes to give mercury and oxygen.
2. Potassium nitrate on heating gets converted into molten potassium nitrite with the release of oxygen.
(b) Potassium chlorate needs heating for quite sometime (to a high temperature) before it decomposes.
Question 4.
What are oxides ? Give two examples for each of me – tallic and non-metallic oxides.
Answer:
Oxides are binary compounds formed by the chemical combination of a substance metal or a non-metal with oxygen.
Examples :
-Metal:
1. Sodium oxide (Na2O).
2. Calcium oxide (CaO).
-Non-metal:
1. Sulphur dioxide (SO2).
2. Carbon dioxide (CO2).
Question 5.
Name the three types of oxidation processes. In which of these large amount of heat and light energy are produced?
Answer:
Oxidation can be categorised into three types :
1. Spontaneous oxidation
2. Rapid oxidation
3. Slow oxidation
Out of the above said three types, rapid oxidation produces large amount of heat and light energy.
Question 6.
What do you observe when the following substances are heated and then tested with moist blue and red litmus – paper?
(a) Sulphur
(b) Phosphorus
(b) Calcium
(d) Magnesium
Answer:
(a) Sulphur : Blue litmus turns red.
(b) Phosphorus : Blue litmus turns red.
(c) Calcium : Red litmus turns blue.
(d) Magnesium : Red litmus turns blue.
Question 7.
Complete and balance the following chemical equations.
Question 8.
(a) Give four uses of oxygen.
(b) How is oxygen naturally renewed in air ?
Answer:
(a) Uses of oxygen
i) Oxygen is used by firemen, miners, aviators, sea divers and even by every living being.
ii) Oxygen is necessary for burning of fuels.
iii) Oxygen mixed with hydrogen as fuel produces.a flame with a very high temperature about 2800°C.
iv) As a fuel in spacecraft.
(b) All living beings use atmospheric oxygen in breathing and burning of fuels and in the formation of oxides of nitrogen. Yet amount of oxygen in the air remains more or less constant. This is because green plants return oxygen to the atmosphere by the process of photosynthesis.
Question 9.
(a) What is rust ?
(b) State at least two ways of prevent rusting.
Answer:
(a) Rust: Rust is hydrated ferric oxide which forms a brownish red coating over iron.
(b) Two ways of prevention of rusting : (Write any two)
i) Painting with red lead.
ii) Oil paint is applied on doors and windows.
iii) Enamel coating. Enamel is a mixture of iron, and steel with silicates.
iv) Coal tar it is used for coating the lower parts of ships and bridges.
Question 10.
State two differences between : Rusting and burning.
Answer:
Difference between rusting and burning:-
1. Rusting is the process in which iron slowly reacts with oxygen in the air and produces a flaky substance called rust while Burning is fast oxidation process in which large amount of energy is produced.
2. Air and moisture are necessary for rusting while Only air is necessary for burning.



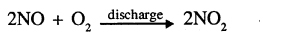





Comments
Post a Comment
This site is all about helping you kids study smart because for Gen Z, studying "hard" is not enough. If you feel there is any way I could improve my posts or if you have any random suggestion that might help make this more kid friendly, please don't hesitate to drop in a comment!
Be sure to check back for my response if you've asked me a question or requested a clarification through the comment section because I do make every effort to reply to your comments here.